UD and Blue Eye Soft — a company led by a UD graduate — were able to execute an agreement on the technology in less than three days, an “unprecedented” speed, the university said.
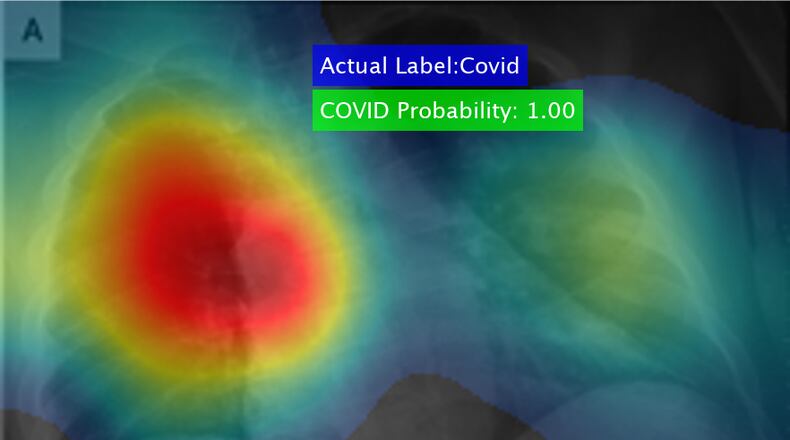
The technology, which detected the presence of the COVID-19 disease in chest X-rays in seconds with 98 percent accuracy, was adapted from existing medical diagnostic software in a matter of hours, UDRI said.
Blue Eye Soft owner Srikanth Kodeboyina and his team further developed the technology, and he plans to submit a full proposal to the U.S. Food and Drug Administration for approval “within a matter of days,” UDRI said in a release. A provisional patent has already been filed.
Credit: Contributed
Credit: Contributed
Kodeboyina is a UD 2011 engineering graduate.
“We hope to be able to bring this new tool to market very quickly,” Kodeboyina said in the release, adding that his start-up company’s staff of 40 employees has been “virtually” joined in the last several days by more than 100 professionals based in Singapore, India and across the U.S., contributing expertise in a variety of fields.
The software, developed by UDRI research scientist Barath Narayanan, uses a “deep learning” algorithm that searches for markings on X-rays that indicate the presence of COVID-19, the university said.
“Narayanan, who spends his days working on sponsored research programs in artificial intelligence for manufacturing and other commercial applications, has for several years been working evenings and weekends pursuing his principal passion: advancing research in AI (artificial intelligence) to help doctors diagnose and treat patients more quickly,” UDRI said in its release.
MORE: AFRL at Wright-Patt unveils drone-killing battlefield laser
Using medical imaging including X-rays, CT scans, blood smear slides and eye scans, Narayanan had already developed a number of software codes that successfully detect—with 92 to 99 percent accuracy—lung and breast cancers and other conditions.
“I wanted to do something for the common good, and medical imaging seemed a good way to do that,” Narayanan said. “Software-based diagnostic tools can serve as a valuable, virtual second opinion for medical professionals, especially in parts of the world where medical teams are short-staffed.”
“I launched Blue Eye Soft in 2017 with a mission to create job opportunities in areas that are innovative and impactful,” Kodeboyina said in UDRI’s announcement. “Right now one of the most pressing needs on the planet is addressing the COVID-19 crisis. Artificial intelligence can be an important solution to support the health care industry.”
In the United States, end-users of the technology will include hospitals, laboratories and medical professionals, he added.
UD Vice President for Research John Leland said UD has been working to execute relevant technology licenses quickly.
“We were driven to help Blue Eye Soft make this technology available as quickly as possible,” Leland said. “We are also excited that a UD grad has licensed one of our technologies and is working diligently to provide medical professionals a new tool in the fight against the spread of this devastating disease.”
About the Author