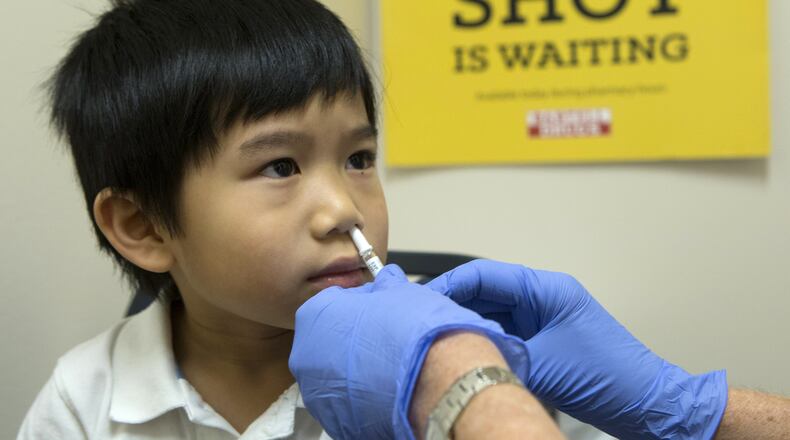
The CDC’s Advisory Committee on Immunization Practices did not recommend FluMist during the 2016-2018 flu seasons because it had shown to have poor efficacy in prevention over the injectable vaccine with H1N1.
FluMist manufacturers say they have seen positive study results of the nasal spray vaccine after they added a new H1N1 strain to the formulation.
Jacobson reminds everyone that this year’s influenza season remains active. He says if you have not received your flu shot this year, do so now.
About the Author